of2 bond angle
Accordingly the bond angle of given compounds are as follows. What is the bond angle in OF2.

Of2 Lewis Structure Molecular Or Electron Geometry Polar Or Non Polar
The bond angles are slightly less than 120 slightly less than 1095 and 180 respectively.
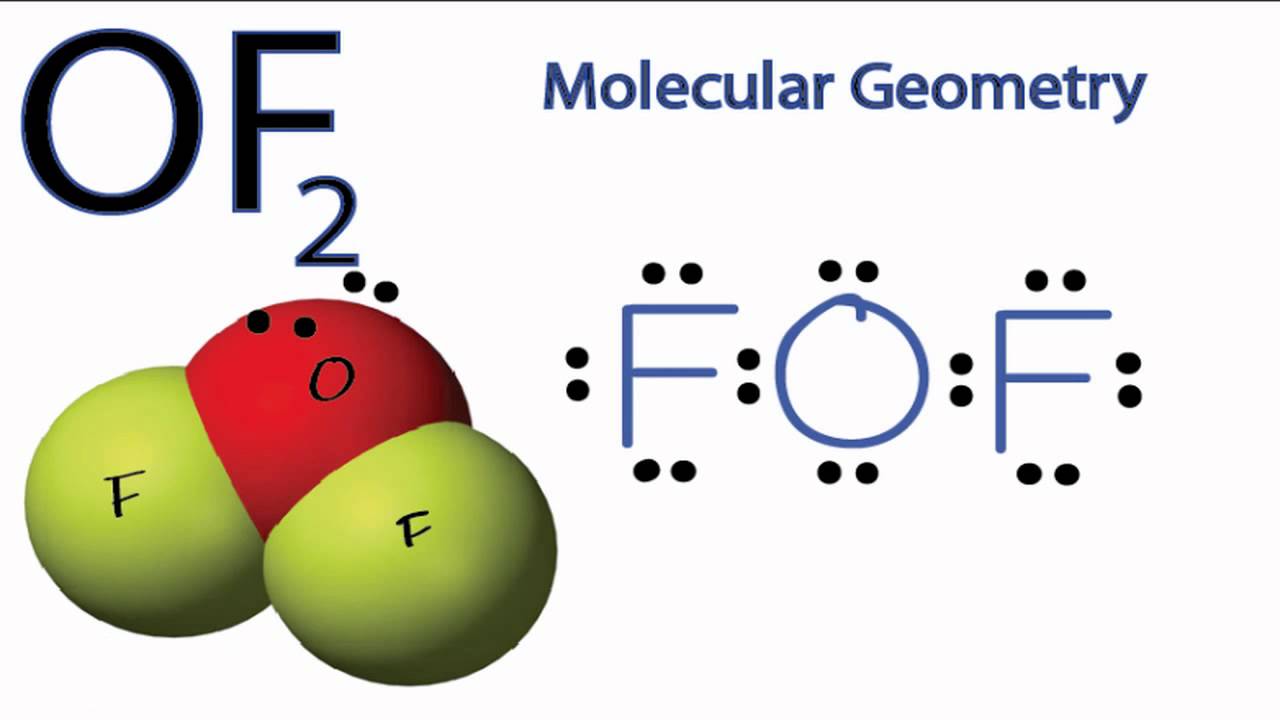
. Therefore the bond angle order is. According to the above chart we have a bent molecule structure. OF2 The electron geometry is tetrahedral and the molecular shape is bentThe theoretical bond angle is 1095 but repulsions by the lone pairs decrease the bond angle to about 103. OF2 has a bent shape and a tetrahedral electron geometry.
In NH3 the bond angles are 107 degrees. Chemistry questions and answers. OF2 PF3 PF4 c. Bond pair-Bond pair repulsions are larger if the electron cloud is nearer to the central atom.
BbNSF The structure of NSF is There is a lone pair on the N atom a lone pair on the S atom and there are three lone pairs on the F atom. Maybe because the flourines are the most electronegative atom in periodic table so they pull each other closer making F-O-F bond angle smaller then. PF3 PF4 OF2. From the values it is inferred that the compound with largest bond angle is and its bond angle value is 112.
OF2 PF3 PF4 a b a с d e. PF4 PF3 OF2 e. OF2 Molecular Geometry note. Hybridization In chemistry an orbit is a fixed path around the nucleus in which electrons tend to move or revolve.
IBr-2 The electron geometry is trigonal bipyramidal. And have 2 lone pairs but ClO 2 has only 3 free electrons. The molecular geometry of NO2- is Use VSEPR to justify your answerA bent bond angle - 109B trigonal planarC linearD bent bond angle - 120. Which one of each of the following has the smallest bond angle.
The bond angle of OF2 is 103 degrees while water is 104 degrees. Which one of each of the following has the smallest bond angle. Is OF2 bent or linear. OF2 PF4 PF3 f.
If playback doesnt begin shortly try restarting your device. PF3 OF2 PF4 d. PF3 OF2 PF4 b. What is bond angle of NH3.
Therefore has highest bond angle. It is close to the tetrahedral angle which is 1095 degrees. Angle of OF 2 is smaller than that of H 2 O as bond angle is inversely to electronegativity. What is the bond angle of OF2.
All of these molecules have two bond pairs and two lone pairs. Precise bond angle is 1031 Watch later. When the bond angle values of the given compound are arranged based on increasing order it gives. So is H2S polar or nonpolar.
The bond angle of OF2 is 103º because two lone pairs on the central atom Fluorine decrease the value bond angle of OF2 from its normal value. The electron cloud in OF 2 will be shifted much towards the Fluorine atom. The bond angle of Oxygen difluoride is 103º. CO2 OF2 BH3.
The actual bond angle for H 2 O is 1045 while the angle for OF 2 is 1031. The central oxygen atom has two lone pairs of electrons and the bond angle of F-O-F is 109 27. OF2 PF4 PF3 e. PF4 PF3 OF 2 C.
The electron geometry of OF2 is tetrahedral and molecular geometry is Bent. Oxygen is more electronegative than hydrogen. Furthermore What is the molecular geometry and molecular polarity for OF2 Therefore the shape os OF2 is changed to bent shape. PF4 OF2 PF3 d.
See the answer See the answer See the answer done loading. The VSEPR notation for the OF2 molecule is AX2E2. Which of the following lists is correctly arranged in order of increasing bond angle about the central atom. It is expected that the bond angles of the molecules should not be ideal and should deviate from 1095 because VSPER theory Valence shell electron pair.
The lone pairs occupy the equatorial positions with the Br atoms in the axial locations. CO2- Bond angles are 180 degrees. In Cl2O one of the Cl atoms would be the central atom instead of the O atom because Cl is less electronegative than O unlike in OF2 where the O is less electronegative than the F atoms and thus the central atom. It has a linear molecular geometry and sp3 hybridization.
The bond angle is around 103 degrees due to the repulsion of the lone pair. According to the above chart we have a bent molecule structure. Show transcribed image text Expert Answer. Its a direct result of Dragos Rule.
Why is the bond angle in H2Ogreater than in OF2. Oxygen difluoride OF2 or F2O CID 24547 - structure chemical names physical and chemical properties classification patents literature biological activities. The oxygen atom in OF2 The electron cloud geometry would be tetrahedral with a molecular shape of angular and a bond angle of around 104 o. The bond angle is around 103 degrees due to the repulsion of the lone pair.
Why of2 has less bond angle than h2o. PF4 OF2 PF3 b. F is more electronegative than H the electrons in the O-F bond spend more time away from the O and close to the F than the electrons in the O-H bond. The oxygen difluoride OF2 has a bent molecular shape and also have lone pairs on Oxygen and Fluorine atoms.
CO2 OF2 BH3. This small difference is due to the electronegativity of the atoms involved in the substances. The theoretical bond angle is 120 but repulsion by the lone pairs decreases the bond angle to about 117. What is the bond angle of CO2.
This causes the bonding pair electrons to move towards O which results in electron repulsion and a larger bond angle. The bond angle order for the series OF2 H2O OCl2 is OCl2 OH2 OF2. The VSEPR notation for the OF2 molecule is AX2E2. This is an AX_2E molecule so the electron geometry is trigonal planar and the molecular shape is bent.
PF3 OF2 PF4 a. Is OF2 bent or tetrahedral. In the Lewis Structure of OF2 both Fluorine atoms share a single bond with the Oxygen. Yes your analogy that bond angle of OF 2 SF 2 is correct.
Place the following in order of increasing F-A-F bond angle where A represents the central atom in each molecule. Is H2S polar or nonpolar. All these are sp 3 hybridized. By signing up youll get thousands of step-by-step solutions to your homework questions.
This problem has been solved.

What Is The Bond Angle Of Of2 Study Com
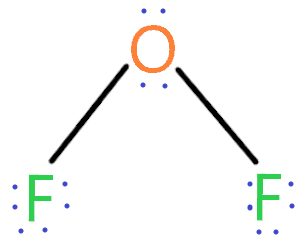
Of2 Lewis Structure Molecular Or Electron Geometry Polar Or Non Polar

Of2 Molecular Geometry Note Precise Bond Angle Is 103 1 Youtube

What Is The Bond Angle Of Of2 Study Com

Of2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Komentar
Posting Komentar